News

- Blog Post
Achieving Calibration Compliance in Pharma and Biotech: What You Need to Know
Maintaining compliance in the pharmaceutical and biotech sectors is becoming increasingly complex. Regulatory bodies such as the FDA and EMA impose strict requirements to ensure product safety, data integrity, and patient protection. Non-compliance can lead to severe consequences, including recalls and reputational damage.

- Blog Post
Autoclave Temperature Mapping with Kaye Validator AVS: Ensuring Compliance in Thermal Validation
In today's highly regulated pharmaceutical and biotech environments, temperature mapping is a non-negotiable process to ensure sterility, compliance, and product integrity. At the heart of this operation lies the Kaye Validator AVS, a 21 CFR Part 11 compliant validator engineered specifically for precision-driven validation tasks. As regulatory scrutiny intensifies and digital transformation accelerates, the ability to streamline qualification processes using innovative, wired validation systems like the AVS is becoming mission-critical for QA teams worldwide.
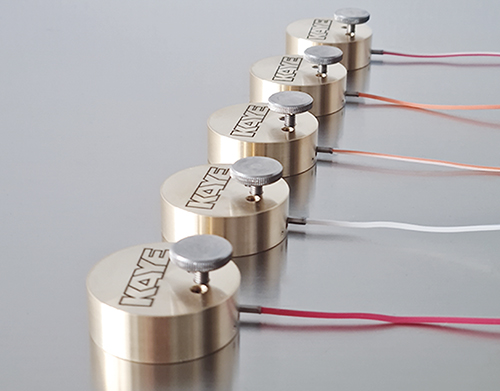
- Blog Post
Measuring Surface Temperature – Professional and Secure
Accurately measuring surface temperature is critical in many controlled environments, particularly in sensitive pharmaceutical and biotechnological processes. However, this seemingly straightforward task becomes increasingly complex in vacuum conditions, such as those found in freeze dryers. Traditional temperature validation methods often fall short, being time-consuming and vulnerable to setup errors. Enter the Kaye Sensor Surface Adapter—a professional and secure solution developed to meet the highest standards of validation and monitoring.

- Blog Post
Sustainable Practices at Kaye: Our Commitment to the Environment
Sustainability is not an option; it is a necessity. As a leading global company in validation systems for the pharmaceutical and biotechnological industries, Kaye, a subsidiary of Amphenol, is committed to shaping a sustainable future. We recognize the crucial role that sustainable practices play in protecting our planet and commit to integrating sustainability into every aspect of our operations.

- Blog Post
Use of Battery-Powered Real-Time Data Loggers at Temperatures Above +140°C
When it comes to high-precision thermal validation in closed chambers—such as depyrogenation tunnels, drying ovens, or high-temperature test environments—real-time data loggers are indispensable tools. They offer unmatched flexibility in handling and placement while enabling continuous monitoring and validation. However, like all technologies, they face limitations—especially when operating in extreme heat.

- Blog Post
Integrity as a cornerstone and standard for ethical conduct
In a time when companies face increasing pressure in a rapidly changing market, integrity is an essential pillar for sustainable success. At Kaye, a company of Amphenol, integrity is not just part of our corporate philosophy but also the standard by which all our decisions and actions are measured.

- Blog Post
Innovation – Our guiding principle for over 65 years
In the dynamic world of technological and automated solutions, innovation is the key to success. At Kaye, a subsidiary of Amphenol, we have embraced this core value for over 65 years. Our continually innovative approaches, which push the boundaries of what is technically possible to develop practical solutions, have established us as a leading provider in the validation of thermal processes. The needs of our users are at the forefront—from developing technologically outstanding products to meeting all regulatory requirements in the pharmaceutical and biotechnology sectors.

- News Post
Precision Certified: Kaye India Laboratory Secures ISO 17025 Accreditation
We’re excited to share a significant achievement — the Kaye India Laboratory in Hyderabad has officially been awarded ISO/IEC 17025:2017 accreditation by IQAS. This milestone marks a vital step forward in our continual efforts to uphold the highest levels of quality, precision, and technical capability in our testing and calibration operations. Receiving ISO 17025 accreditation demonstrates global recognition of our lab’s competency and commitment to delivering reliable and accurate results.

- Blog Post
Diversity as a Success Factor at Kaye
At Kaye, a subsidiary of Amphenol, we recognize diversity as a crucial component of our corporate culture. It fosters creative solutions and drives us to incorporate a global perspective into our daily decisions, which ultimately enhances our success and innovation.

- News Post
INTERPHEX 2025 Wrap-Up: Thank You for Engaging with Kaye
As the doors closed on INTERPHEX 2025, the Kaye team would like to extend our heartfelt thanks to all the attendees, partners, and industry peers who visited our booth during this truly inspiring event. Held from April 1–3 at the Javits Center in New York City, INTERPHEX 2025 brought together nearly 10,000 pharmaceutical and biotechnology professionals for three incredible days of innovation, collaboration, and learning.

