News

- Blog Post
Minimizing Risk when Using a Traceable Temperature Standard
Precision and reliability are of the utmost importance in the validation of thermal processes. These requirements demand time-consuming and complex procedures for the validation, calibration, and verification of the instruments used. In this context, the Kaye IRTD, a high-precision traceable temperature standard, plays a noteworthy role. Its application is crucial for the accuracy of Kaye's validation systems, like the Kaye AVS and the Kaye ValProbe, and thus forms an integral part of these verification and calibration processes. However, the use of temperature standards also brings specific challenges and potential risks. This article shows how such risks can be minimized, and the validation and calibration processes can be optimized and have their risks reduced.

- Blog Post
Kaye's Time Warp: Battery-Powered Data Loggers
In this blog post, we would like to focus on the addition to the Kaye product line that occurred in the early 2000s: The integration of wireless, battery-powered data loggers, an aspect that was hinted at in our whitepaper on wired vs. wireless validation systems.

- Blog Post
Kaye Validation Systems for Rent
There are instances when budgets don't allow for the acquisition of new or supplemental validation systems, or when project requirements unexpectedly change. In these cases, renting additional measurement and validation system capacities can be a practical solution. Our flexible rental models allow the validation equipment to be adapted to meet present validation specifications.

- Blog Post
Kaye's Time Warp: ISO Accreditation
Having delved into the history of the various Kaye measurement systems in previous blog posts, this edition is dedicated to the essential service offerings that go hand-in-hand with Kaye's validation systems. In part 3 of this blog post series, we already addressed the necessity for traceability in calibration. From the very first Kaye validation systems, this traceability was ensured by the Kaye IRTD-400 temperature standard. However, complete traceability in relation to national standards is only ensured by the necessary accreditation. This edition will shed light on what this means in concrete terms.

- Blog Post
Why is Measuring Pressure Important in the Validation of a Steam Autoclave?
In our blog post "Importance of Steam Quality in Moist Heat Sterilization," we already discussed the need to measure temperature and pressure and their interrelationship for proper sterilization results. In this post, we would like to delve deeper into this topic.

- Blog Post
Kaye's Time Warp: Ice Point and Cold Junction Compensation
The loyal readership of our blog post series “Kaye’s Time Warp” might wonder about the significance of the Kaye Ice Point Reference in the context of validating thermal processes in the GxP environment. In fact, Kaye Ice Point references are more likely to be found on aluminum furnaces, in turbine test stands, or in power plant control rooms. However, the basic knowledge that our company founder Dr. Joseph Kaye acquired in the early 1950's in this context is crucial for the development of high-precision validation systems based on thermocouples as temperature sensors.

- Blog Post
Importance of Steam Quality in Moist Heat Sterilization
In this blog post, we would like to delve a bit deeper into the importance of steam quality in moist heat sterilization. As usual in our blog posts, we do not lay claim to a highly scientific presentation, but rather focus on imparting basic knowledge. For further in-depth understanding, there's plenty of specialized literature available to interested readers.

- Blog Post
Kaye's Time Warp: The Traceable Temperature Standard
Part 3 of this blog post series deals with another important part of the measurement philosophy that Kaye established early on as a system supplier for measurement systems for the validation of thermal processes. As early as 1972, with the introduction of the first Kaye Validator Digistrip I, it was clear that a data recorder or data logger (Blog Post Part 1) for data acquisition and the calibration furnaces and baths described (Blog Post Part 2) were only part of the journey towards an automated validation system.
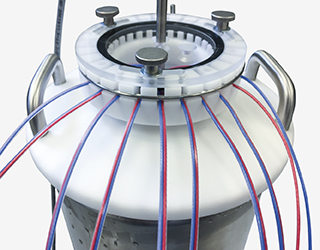
- Blog Post
Thermal Validation of Cryo Containers in the GxP Environment
Cryo containers are found in various applications in the pharmaceutical and biotechnology industries. Be it to store biological samples like cells and tissues at extremely low temperatures to preserve them for future studies to use, or even for transportation. Thus, cryo containers are part of the cold chain and therefore must be qualified and validated according to EU GDP (European Union Good Distribution Practice). These guidelines were developed to ensure the quality of pharmaceuticals throughout the entire distribution process from manufacturing to consumption.

- Blog Post
Kaye's Time Warp: From the Bulky Block Calibrator to the Flexible Calibration Unit
The calibration of the systems and sensors currently in use, along with the complete elimination of identified deviations, constitute fundamental components of a qualification. These are essential for the subsequent validation of a critical process within a GxP context.

