News

- Blog Post
What Added Value Does Real-Time Data Logging Deliver? The Modern Validation Standard
In many pharmaceutical and biotech facilities, validation teams still rely on conventional data loggers paired with reader stations. The logic is familiar. If a system has worked reliably for years, it feels sufficient. However, this assumption often conceals inefficiencies that only surface when teams compare legacy workflows with modern, real-time validation systems.

- Blog Post
IQ, OQ, PQ: What Each Phase Means in Pharmaceutical and Biotech Equipment Validation
Pharmaceutical and biotech manufacturers depend on precise, reliable, and fully qualified equipment to protect product integrity, patient safety, and regulatory compliance. IQ, OQ, and PQ offer a structured path to proving that systems operate exactly as intended. IQ, OQ, and PQ represent three core phases of equipment qualification designed to verify installation accuracy, operational consistency, and performance reliability. Each stage builds upon the last, forming a structured framework that supports process qualification and long-term compliance. Together, they create a documented assurance trail that demonstrates equipment is fit for use throughout its lifecycle.

- Blog Post
Dry‑Block Calibrator vs. Temperature Calibration Bath: A Practical Selection Guide
In pharmaceutical and biotech validation, precise temperature calibration is fundamental to quality assurance. Every activity that depends on accurate thermal measurements, from sterilization cycles to stability studies, requires calibration equipment that ensures traceability and reliable performance. Two commonly used references are dry‑block calibrators and liquid temperature baths. Both verify sensor accuracy, but they operate differently and suit different use cases. This comparison explains how each method works and where it performs best in regulated environments, with selection driven by probe geometry, required uncertainty, throughput, and whether calibration is performed in‑lab or on‑site.

- Blog Post
Why Pharma & Biotech Should Be Using Database-Driven Thermal Validation Systems
In the pharmaceutical and biotech industries, maintaining product integrity is non-negotiable. Thermal validation ensures that temperature-sensitive products—such as vaccines, biologics, and sterile drugs—are stored, processed, and transported under validated conditions. Modern thermal validation software and hardware, when paired with robust database management systems, provide companies with scalable, compliant, and future-proof solutions that outperform traditional methods.

- Blog Post
Temperature Calibration in Pharma: Why It Matters and How to Do It Right
In pharmaceutical manufacturing, precision is the foundation of safety, efficacy, and regulatory compliance. Each measurement - whether related to temperature, humidity, or pressure - directly impacts product quality and ultimately patient health. Calibration ensures that instruments perform within defined tolerances, aligning results with recognized calibration references. Without regular calibration, measurement drift can compromise data integrity, trigger compliance violations, and endanger product reliability.

- Blog Post
Environmental Monitoring Systems for Pharma, Biotech, and Regulated Industries
Environmental monitoring plays a vital role in ensuring compliance, product integrity, and public safety across regulated industries. Whether in pharmaceuticals, biotech, healthcare, cold storage, or cleanroom facilities, effective monitoring helps organizations maintain validated environments, prevent contamination, and meet global compliance standards.

- Blog Post
Thermal Validation: Ensuring Sterility in Healthcare Pharmaceutical and Food Manufacturing
The assurance of sterility is fundamental in healthcare, pharmaceutical manufacturing, and food processing. Contamination of the products are not only a threat to patient safety and public health but also a significant source of economic liability due to product recalls and regulatory sanctions. Within this context, thermal validation plays a critical role in confirming that sterilization processes are consistent, effective, and compliant with international standards.

- Blog Post
Warehouse Mapping: Ensuring Temperature Integrity Across Critical Environments During Winter
As winter approaches, temperature-controlled environments across industries, from pharmaceuticals and biotechnology to laboratories, logistics, and food storage, face significant challenges. Cold temperatures, fluctuating humidity levels, and uneven heating can jeopardize product stability and regulatory compliance. To address these seasonal risks, organizations rely on warehouse mapping during winter, a precise validation process designed to confirm that environmental conditions remain consistent and compliant throughout the colder months.

- Blog Post
ISO/IEC 17025:2017 – The Global Standard for Calibration Laboratories
ISO/IEC 17025:2017 is the international standard that defines the competence, impartiality, and consistent operation of testing and calibration laboratories. In today’s business and regulatory landscape, accurate, traceable calibration services to meet compliance requirements. Achieving ISO 17025 calibration ensures that laboratories demonstrate both technical proficiency and global credibility. For organizations evaluating calibration labs, this standard is essential in ensuring reliability, regulatory alignment, and long-term trust. The adoption of ISO 17025 is not simply a compliance exercise but a strategic investment in quality and operational excellence.
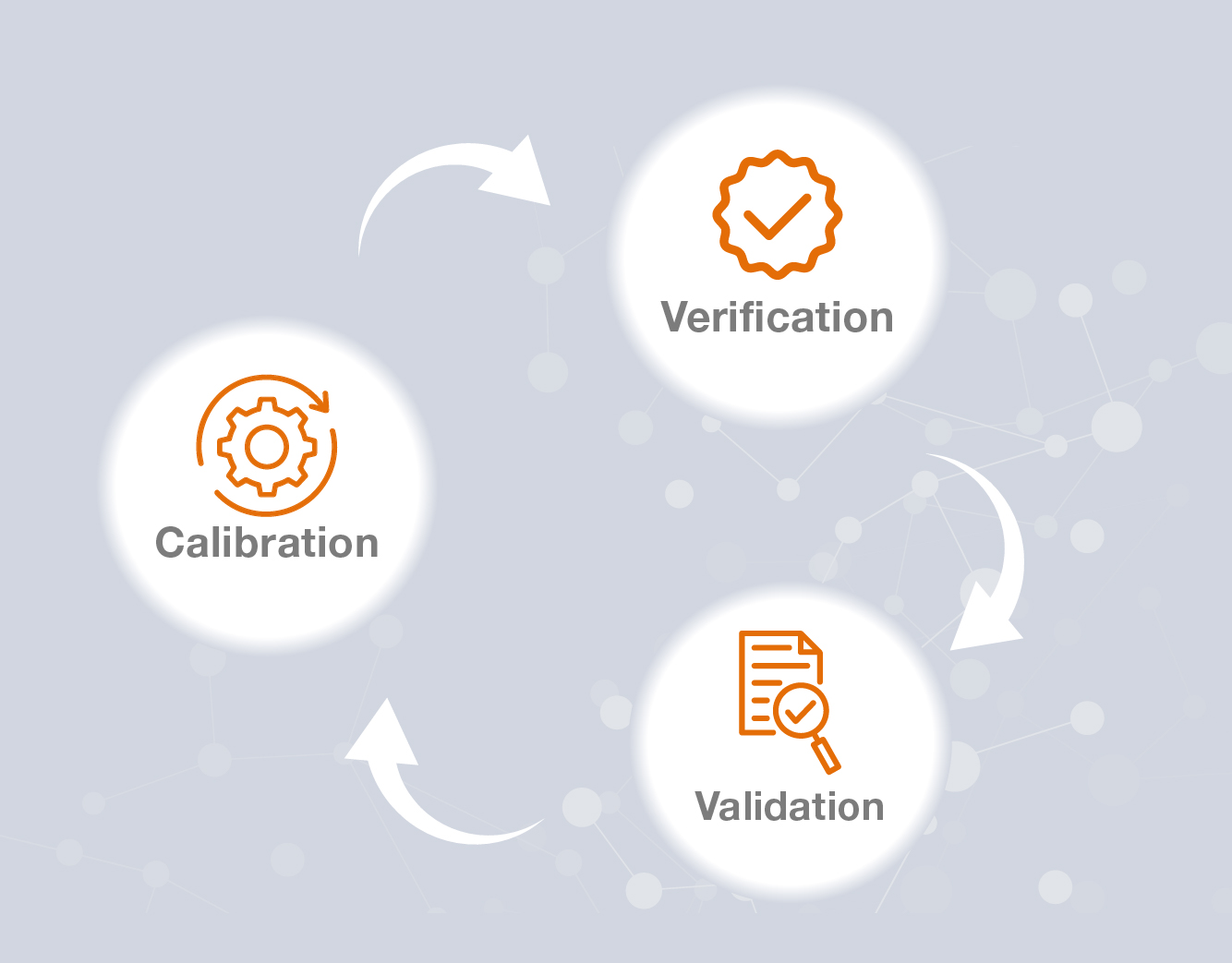
- Blog Post
Calibration, Verification, and Validation Explained: Achieving Accuracy and Regulatory Compliance
In precision-driven industries such as pharmaceuticals, biotechnology, and life sciences, calibration, verification, and validation form the foundation of effective quality assurance. While often misunderstood or used interchangeably, each process serves a distinct role in ensuring measurement accuracy, operational reliability, and regulatory compliance. Understanding how they differ—and work together—is essential for maintaining consistency, safety, and efficiency across operations.

