News

- Blog Post
What Added Value Does Real-Time Data Logging Deliver? The Modern Validation Standard
In many pharmaceutical and biotech facilities, validation teams still rely on conventional data loggers paired with reader stations. The logic is familiar. If a system has worked reliably for years, it feels sufficient. However, this assumption often conceals inefficiencies that only surface when teams compare legacy workflows with modern, real-time validation systems.

- Blog Post
IQ, OQ, PQ: İlaç ve Biyoteknoloji Ekipmanlarının Doğrulamasında Her Aşamanın Anlamı
İlaç ve biyoteknoloji üreticileri, ürün bütünlüğünü, hasta güvenliğini ve mevzuata uygunluğu korumak için hassas, güvenilir ve tam olarak onaylanmış ekipmanlara güvenmektedir. IQ, OQ ve PQ, sistemlerin tam olarak amaçlandığı gibi çalıştığını kanıtlamak için yapılandırılmış bir yol sunar. IQ, OQ ve PQ, kurulum doğruluğunu, operasyonel tutarlılığı ve performans güvenilirliğini doğrulamak için tasarlanmış ekipman onayının üç temel aşamasını temsil eder. Her aşama bir öncekini temel alarak, süreç kalifikasyonunu ve uzun vadeli uyumluluğu destekleyen yapılandırılmış bir çerçeve oluşturur. Birlikte, ekipmanın yaşam döngüsü boyunca kullanıma uygun olduğunu gösteren belgelenmiş bir güvence izi oluştururlar.

- Blog Post
Kuru Blok Kalibratör ve Sıcaklık Kalibrasyon Banyosu: Pratik Seçim Kılavuzu
İlaç ve biyoteknoloji doğrulamasında, hassas sıcaklık kalibrasyonu kalite güvencesi için temel öneme sahiptir. Sterilizasyon döngülerinden stabilite çalışmalarına kadar, doğru termal ölçümlere bağlı olan her faaliyet, izlenebilirlik ve güvenilir performans sağlayan kalibrasyon ekipmanı gerektirir. Yaygın olarak kullanılan iki referans, kuru blok kalibratörler ve sıvı sıcaklık banyolarıdır. Her ikisi de sensör doğruluğunu doğrular, ancak farklı şekilde çalışır ve farklı kullanım durumlarına uygundur. Bu karşılaştırma, her bir yöntemin nasıl çalıştığını ve düzenlenmiş ortamlarda en iyi performansı nerede gösterdiğini, prob geometrisi, gerekli belirsizlik, verim ve kalibrasyonun laboratuvarda mı yoksa sahada mı yapıldığına bağlı olarak seçim yapıldığını açıklamaktadır.

- Blog Post
İlaç ve Biyoteknoloji Endüstrisi Neden Veritabanı Yönetimi ile Güçlendirilmiş Termal Doğrulama Yazılımı ve Donanımına Güvenmelidir?
İlaç ve biyoteknoloji endüstrilerinde ürün bütünlüğünün korunması tartışılmaz bir gerekliliktir. Termal validasyon, aşılar, biyolojik ürünler ve steril ilaçlar gibi sıcaklığa duyarlı ürünlerin doğrulanmış koşullar altında depolanmasını, işlenmesini ve taşınmasını sağlar. Modern termal validasyon yazılımı ve donanımı, sağlam veritabanı yönetim sistemleriyle birleştirildiğinde, şirketlere geleneksel yöntemlerden daha üstün, ölçeklenebilir, uyumlu ve geleceğe dönük çözümler sunar.

- Blog Post
İlaç Sektöründe Sıcaklık Kalibrasyonu: Neden Önemlidir ve Nasıl Doğru Yapılır?
İlaç üretiminde hassasiyet, güvenlik, etkinlik ve mevzuata uygunluğun temelidir. Sıcaklık, nem veya basınçla ilgili her ölçüm, ürün kalitesini ve nihayetinde hasta sağlığını doğrudan etkiler. Kalibrasyon, cihazların tanımlanmış toleranslar dahilinde çalışmasını sağlayarak sonuçları tanınmış kalibrasyon referanslarıyla uyumlu hale getirir. Düzenli kalibrasyon yapılmazsa, ölçüm sapmaları veri bütünlüğünü tehlikeye atabilir, mevzuata uygunluk ihlallerine yol açabilir ve ürün güvenilirliğini tehlikeye atabilir.

- Blog Post
İlaç, Biyoteknoloji ve Düzenlemelere Tabi Sektörler için Çevresel İzleme Sistemleri
Çevresel izleme, düzenlemelere tabi endüstrilerde uyumluluk, ürün bütünlüğü ve kamu güvenliğini sağlamada hayati bir rol oynar. İlaç, biyoteknoloji, sağlık hizmetleri, soğuk hava depoları veya temiz oda tesislerinde olsun, etkili izleme, kuruluşların onaylanmış ortamları korumasına, kontaminasyonu önlemesine ve küresel uyumluluk standartlarını karşılamasına yardımcı olur.

- Blog Post
Termal Doğrulama: Sağlık, İlaç ve Gıda Üretiminde Steriliteyi Sağlama
Sterilitenin sağlanması, sağlık hizmetleri, ilaç üretimi ve gıda işleme alanlarında temel öneme sahiptir. Ürünlerin kontaminasyonu, hasta güvenliği ve halk sağlığı için bir tehdit oluşturmakla kalmaz, aynı zamanda ürün geri çağırma ve yasal yaptırımlar nedeniyle önemli bir ekonomik yük oluşturur. Bu bağlamda, termal validasyon, sterilizasyon süreçlerinin tutarlı, etkili ve uluslararası standartlara uygun olduğunu doğrulamada kritik bir rol oynar.

- Blog Post
Depo Haritalama: Kış Aylarında Kritik Ortamlarda Sıcaklık Bütünlüğünün Sağlanması
Kış yaklaşırken, ilaç ve biyoteknolojiden laboratuvarlara, lojistikten gıda depolamaya kadar farklı sektörlerdeki sıcaklık kontrollü ortamlar önemli zorluklarla karşı karşıya kalmaktadır. Soğuk sıcaklıklar, dalgalanan nem seviyeleri ve eşit olmayan ısıtma, ürün stabilitesini ve mevzuata uygunluğu tehlikeye atabilir. Bu mevsimsel riskleri ele almak için kuruluşlar, soğuk aylar boyunca çevresel koşulların tutarlı ve uyumlu kaldığını doğrulamak için tasarlanmış hassas bir doğrulama süreci olan kış aylarında depo haritalamasına güvenmektedir.

- Blog Post
ISO/IEC 17025:2017 - Kalibrasyon Laboratuvarları için Küresel Standart
ISO/IEC 17025:2017, test ve kalibrasyon laboratuvarlarının yetkinliğini, tarafsızlığını ve tutarlı çalışmasını tanımlayan uluslararası bir standarttır. Günümüzün iş ve mevzuat ortamında, uyumluluk gereksinimlerini karşılamak için doğru, izlenebilir kalibrasyon hizmetleri gereklidir. ISO 17025 kalibrasyonuna ulaşmak, laboratuvarların hem teknik yeterlilik hem de küresel güvenilirlik göstermesini sağlar. Kalibrasyon laboratuvarlarını değerlendiren kuruluşlar için bu standart güvenilirlik, mevzuat uyumu ve uzun vadeli güven sağlamak açısından çok önemlidir. ISO 17025'in benimsenmesi sadece bir uyumluluk uygulaması değil, kalite ve operasyonel mükemmelliğe yapılan stratejik bir yatırımdır.
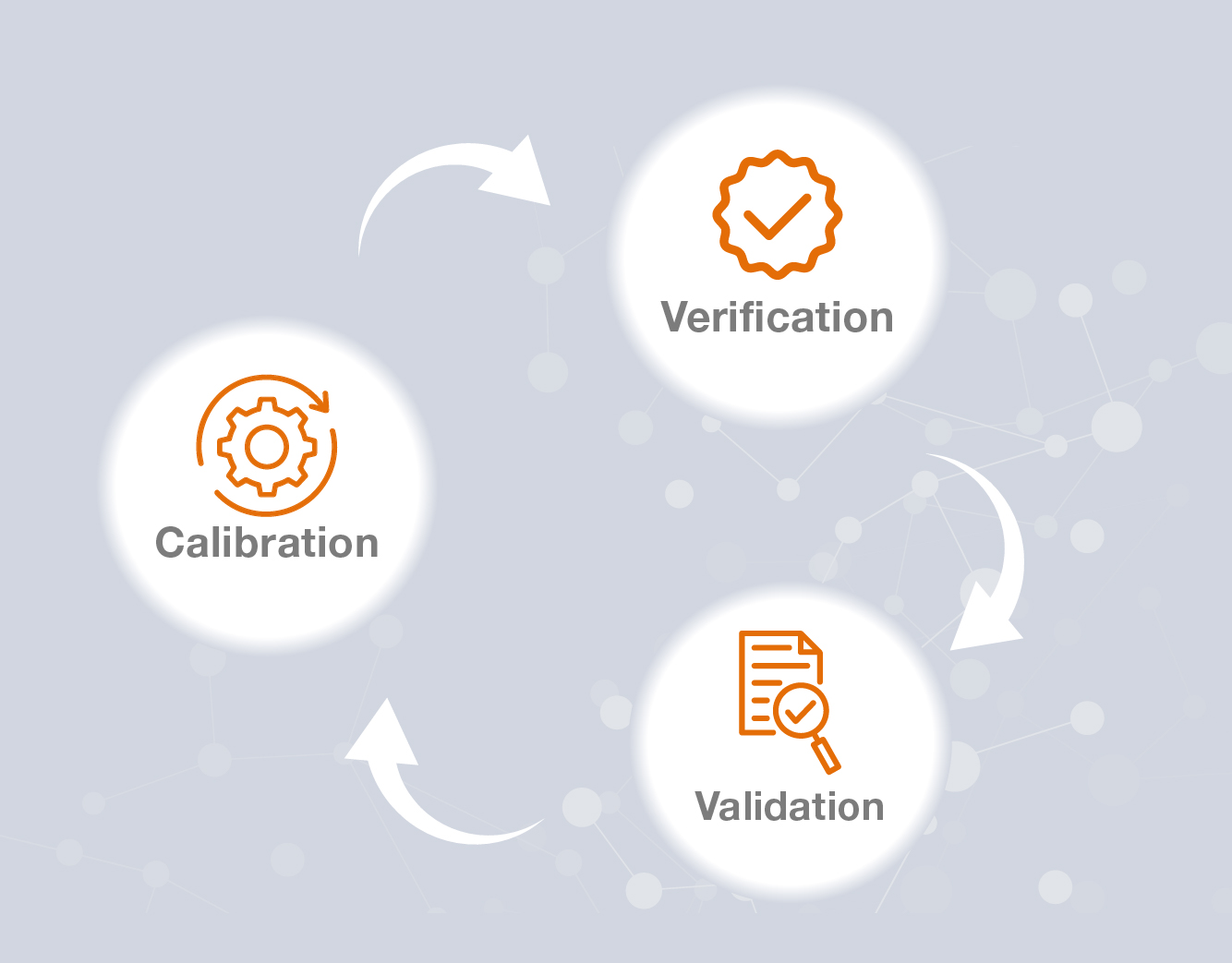
- Blog Post
Kalibrasyon, Doğrulama ve Validasyon Açıklaması: Doğruluk ve Mevzuata Uygunluk Sağlama
İlaç, biyoteknoloji ve yaşam bilimleri gibi hassasiyet odaklı sektörlerde kalibrasyon, doğrulama ve geçerlilik, etkili kalite güvencesinin temelini oluşturur. Genellikle yanlış anlaşılsa veya birbirinin yerine kullanılsa da, her bir proses ölçüm doğruluğu, operasyonel güvenilirlik ve mevzuata uygunluğun sağlanmasında farklı bir rol oynar. Aralarındaki farkı ve birlikte nasıl çalıştıklarını anlamak, operasyonlarda tutarlılığı, güvenliği ve verimliliği korumak için çok önemlidir.

