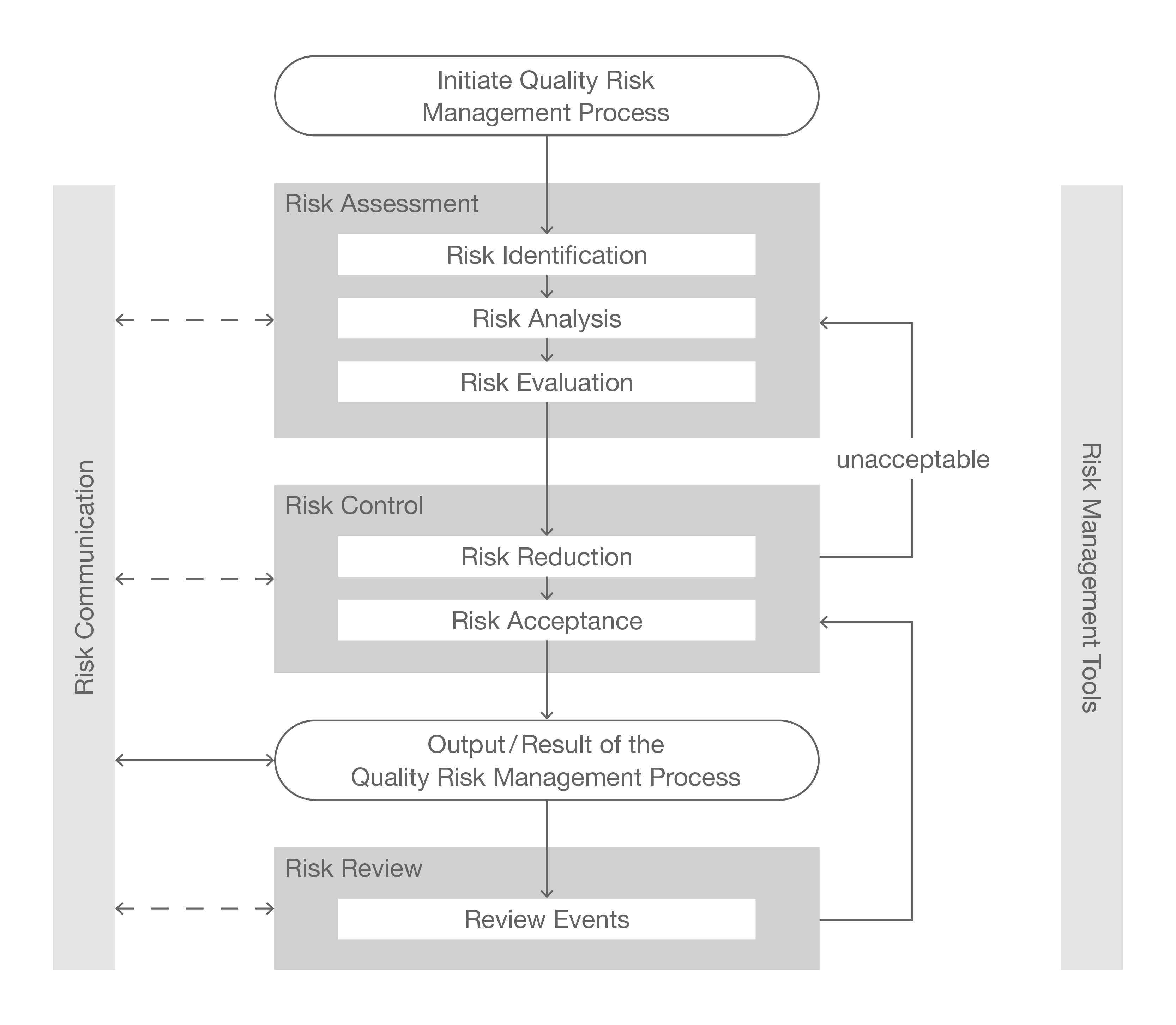
Risk management is a central component in the GxP environment throughout the entire lifecycle of a facility/process/system/instrument and is part of guidelines and regulations. The risk management process aims to ensure that all risks are identified, assessed, and adequately controlled to guarantee the quality and safety of pharmaceutical products. The five fundamental steps of risk management are:
- Risk Identification
- Risk Assessment
- Risk Control
- Risk Communication
- Risk Review